Non-organic semiconductors are used in crystalline form. The definition of a crystal is not that it looks like a gem, though that is a common result, it's that the constituent atoms are arranged in a regular pattern known as a lattice. A crystal of silicon forms a diamond-cubic lattice structure like this:
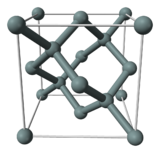
If you look carefully (and understand that this is just one cell; it replicates on all sides) you'll notice that each atom has 4 bonds. Each bond involves one electron from each atom. In normal silicon, some electrons do dissociate from their parent atoms and leave holes behind (this is \$n_i\$, the intrisic carrier concentration). However, the normal pattern is for a bond to more-or-less permanently link an electron and a hole.
The reason that trivalent and pentavalent atoms are acceptors and donors is that this bond structure is maintained when impurities replace atoms in this lattice. If you add a trivalent atom to this structure, you still require four bonds but you only have three valence electrons to work with. This is an acceptor. Each trivalent acceptor atom (in a unit volume) increases \$N_A\$ by one. Silicon cannot be a donor because it only has four valence electrons; if it donates an electron to fill this bond then that leaves a hole on the silicon atom. If you add a pentavalent atom to the structure, four of its electrons will form bonds but the fifth cannot form a bond. It becomes a donor, and each pentavalent donor atom increases \$N_D\$ by one.
If you added an impurity with two or six valence electrons, the same thing would happen, but two electrons or two holes would be added per atom. However, this is more likely to cause a break in the crystal structure and isn't really very common in industry as far as I know.
It sounds like you want to use something similar, yet inferior, to laser trimming. Aging is a non-linear process and depends on your substrate and doping. Not only that, but typical accelerated aging processes (by heating) can take days. For instance, the graph below from this paper

That's BaTiO3 with different dopants.
It's hard to find much information about this, likely because it's not a very great idea.

Best Answer
Yes you are correct, the semiconductor stays in the intrinsic state.
By applying temperature, some electrons acquire a state of excitement, which causes them to jump to the conduction band from the valence band. This increase of electrons in the conduction band corresponding to a decrease of the resistivity of the material.